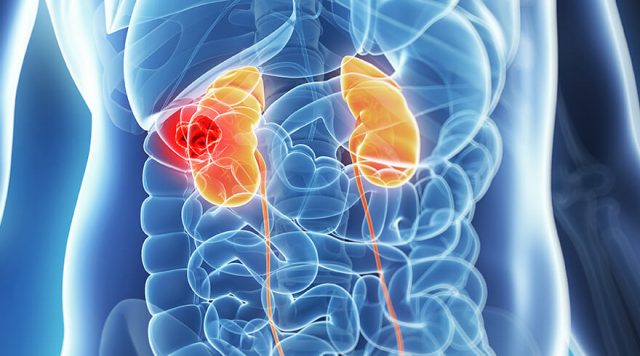
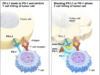
A study by Roche arrived in a conclusion that a combination treatment of Tecentriq (atezolizumab) and Avastin (bevacizumab) decreases the risk of worsening of advanced kidney cancer patients’ disease.
Tecentriq is an antibody engineered to bind to PD-L1, and is already approved globally to aid in the treatment of specific types of metastatic urothelial and metastatic non-small cell lung cancers. Avastin, on the other hand, is currently the only available cell carcinoma treatment that aims vascular endothelial growth factor (VEGF) which is a protein involved in the maintenance and formation of blood vessels.

The Swiss pharma conglomerate demonstrated the results last February 10 at the Genitourinary Cancers Symposium, which was held in San Francisco.
Comparison of the efficacy and efficiency of Tecentriq and Avastin with the standard treatment Sutent (sunitinib) was done during the IMmotion151 trial (NCT02420821) on patients inoperable, locally advanced or metastatic renal cell carcinoma (mRCC).
There were a total of 915 participants who didn’t undergo prior experimental or systematic therapy.
Intravenous doses of 1,200 mg and 15 mg/kg of Tecentriq and Avastin were given to the patients every three weeks. Participants in the Sutent group were administered with an oral dose of 50 mg once daily for four weeks, followed by two weeks of rest. Treatments were continuously administered until no clinical benefit or unacceptable toxicity.
Primary endpoints were assessed by the researchers namely: 1) progression-free survival (PFS) in patients with detectable PD-L1 expression (which may allow tumors to escape the body’s immune response), and 2) overall survival in the whole group, until up to sixty-three (63) weeks of treatment.
Patients who were given Tecentriq along with Avastin had a twenty-six (26) percent lower risk of the cancer worsening or death (PFS) than those in the Sutent group. Although the results are promising, these are still preliminary.
According to the subgroup results, treatment with Tecentriq and Avastin delayed disease progression across all risk factor groups (poor, intermediate, favorable) in patients with detectable tumor PD-L1 expression.
Results on the safety criteria of the combination treatment were the same with those of the individual medications as well as to the results of the Phase 2 IMmotion150 study (NCT01984242). With this, the rate of the life-threatening adverse events and treatment-related severe events was lower with the combination approach (40%) than with Sutent (54%).
The combination treatment of Tecentriq and Avastin remarkably delayed the time to worsened symptoms that hinders day-to-day life in the overall study group of patients.
In a press release, chief medical officer and head of global product development at Roche/Genetech, Dr. Sandra Horning, shared that this is the second positive Phase 3 study that includes the combination of Tecentriq and Avastin as an inclusion in the treatment routine. The next stop for their company is the plans of discussing these results with European Medicines Agency and the U.S. Food and Drug Administration.